 Phage Therapy
Phage Therapy
Thomas Kalinoski
Abstract
Phage therapy
is the application of bacteriophages to therapeutic
effect in the body to control pathogenic bacteria. The use of phages has many
potential applications for treating bacterial infections, especially infections
that do not respond to conventional antibiotic treatment. Although the use of
phages to control bacteria has been proposed decades ago, phage therapy use for
human medicine has not yet been approved in most of the world and continues to
undergo extensive research. This
review examines various research literatures involving the use of phages in
human medicine. Included background information features some of the basic
information already known about the bacteriophages
and their potential applications. The study’s results reveal the findings on
the efficacy of phage therapy as well as the present concerns about its safety
and conclusions about its potential future in western medicine.
Introduction
Even from
the start of widespread antibiotic use in the 1940’s it has been known that
resistance strains of bacteria would become a problem in treatment of
infections. Today, there are a diminishing number of antibiotics that can be
used in many cases, and highly resistant strains are becoming more and more of
a problem to health care professionals. Discovery of completely novel
antibiotics is rare, and the development of more effective antibiotics cannot
keep up with resistance. Therefore, use of bacteriophages
to treat infections stands out as a promising alternative. Research on bacteriophages to treat specific diseases is widespread,
and this review was conducted in an effort to amass this research and analyze
its significance. The study brings together a variety of different research
literature in order to review the critical points of current knowledge on phage
therapy. In this review, the efficacy
of phage therapy will first be examined, then the present concerns regarding
safety of its use in humans. Finally, this review will address the issues and
obstacles that need to be overcome for this technology to take hold in western
medicine.
Background
Bacteriophages have been studied for many decades, resulting in a wealth
of information on the topic. Bacteriophages,
or simply phages, are viruses that exclusively infect bacterial cells. Bacteriophages were discovered in the early 20th
century, and not long after their discovery, they were already being tested
therapeutically to fight against infections. The first study was conducted by
Felix d'Hérelle at the Hôpital
des Enfants-Malades in Paris in 1919 on a 12 year old boy with dysentery (10). According to the
report, the boy’s symptoms ceased after a single administration. In 1923,
George Eliava founded the Eliava
Institute in Georgia, devoted to the development of phage therapy. Research and
commercialization of phages soon began in Russia and the United States in the
1940’s, at the same time that antibiotics were taking off. The fact that early
use of phage therapy was largely unreliable, and that the newly discovered
antibiotics were seen as “wonder drugs”, ensured a loss of interest from most
of the scientific community. Research into phage therapy continued in the
Soviet Union, where it was isolated due to the cold war. Phage therapy
continues to this day exclusively in Georgia at the Eliava
Institute (8). A renewed interest to find alternative methods of treating
bacteria due to increasing antibiotic resistance has increased the amount of
recent research on the subject in the west. The fact that bacteriophages
are much more specific agents than antibiotics, have few side effects, do not
stress the liver, and are self-replicating, make phage therapy an attractive
option (1).
Effectiveness of Phage Therapy
Lytic Activity
Carmody and colleagues found that phage therapy was effective in
the treatment of pulmonary infections due to species of Burkholderia cepacia in a study on mice (3). This
study used the B. cenocepacia
strains AU0728 and K56-2 isolated from sputum of patients with cystic fibrosis
(CP), which were introduced to mice via tracheotomy. The mice were treated with
B. cepacia specific
phage twenty-four hours after infection by either intranasal inhalation or intraperitoneal injection. Bacterial densities in the lungs
were determined seventy-two hours after infection. The treatment of infected
mice with phage via intraperitoneal injection
resulted in significant reduction in lung bacterial density after forty-eight
hours relative to mock treated mice.
In studying
the treatment of Klebsiella pneumonia mediated lobar pneumonia in
mice, Chhibber and colleagues found that phage
therapy can be effective, but the results vary significantly depending on
treatment time (4), suggesting that there is a limited bacteria population
size where use of phages can effectively check its growth. The study used phage
SS, a phage specific for K. pneumonia.
The phage preparation was injected by intraperitoneal
route to mice immediately after infection in one group, and at 6 hours and 24 hours
post-infection in two additional groups. The results showed that phage therapy
has the potential to check the growth of K.
pneumonia in the respiratory tract if initiated at an appropriate time, but
a delay of even six hours rendered the treatment ineffective.
In their
study on the application of phage therapy to chronic bacterial prostatitis (CBP), Letkiewicz and
colleagues found that phage therapy can be used to successfully treat resistant
infections in tissues that are not readily accessible by antibiotics (7). This
article is significant because it is one of few recent studies on
phage therapy in humans. Conducted at the Institute of Immunology and
Experimental Therapy in Poland, experimental phage therapy was tested on
drug-resistant bacterial infections. After E.
faecalis was cultured from infected prostate
fluid, three patients suffering from CBP were treated rectally with a phage
preparation active against the bacteria two times daily for 28-33 days.
Treatment caused bacterial eradication, as well as reduction in prostate size
and pain and significant increases in the maximum urinary flow in all cases.
Immunomodulary
Activity
There is
also evidence in many studies that use of phages for the therapeutic control of
bacterial infections influences the response by the immune system to fighting
the infection. In addition to their role in the direct clearance of bacteria,
many studies also document changes in cytokine levels, leukocyte recruitment, and
antibody production.
In an
article documenting the efficacy of phage therapy in the immunocompromised
host, Borysowski and colleagues describe two major
mechanisms of therapeutic effect due to phage therapy (2). The first relies on
direct killing of bacterial cells by the lytic cycle,
while the other depends on inducing an antibacterial immune response by either
the phage particles themselves or constituents of dead bacterial cells, such as
lipopolysaccaride (LPS). LPS, also called endotoxin, is a component of some bacterial cell walls and
a powerful stimulator of the immune system.
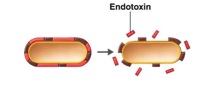
Figure 1. Endotoxin Release upon Cell Lysis.
Retrieved from <http://www2.raritanval.edu>.
This study
pointed out several experiments performed on immunocompetent
mice that suggest direct killing of bacteria as the major mechanism of
therapeutic effect. These researchers concluded that the only significant
mechanism mediating the therapeutic effects of phage preparations is direct
killing of bacterial cells by phage viruses.
Zimecki and colleagues found significant evidence of enhanced
immune activity due to phages in their study on the prophylactic administration
of bacteriophages in the treatment of Staphylococcus aureus
infection in mice (11). Mice were injected with the immunosuppressant cyclophosphamide (CP) intraperitoneally
and then administered S. aureaus intravenously four days later. S. aureus
specific phages were administered intraperitoneally
30 minutes before infection. The results indicated that the highly elevated
numbers of bacteria in CP treated mice were lowered by application of phages to
values observed in mice not subject to CP treatment (with healthy immune
systems). The results indicated efficient removal of bacteria by prophylactic
administration of specific phages, but also revealed positive effects on the
activity of the immune system.
In their
study on the application of phage therapy to chronic bacterial prostatitis, Letkiewicz and
colleagues found strong experimental evidence that purified phages may have an
anti-inflammatory effect. They have been found to alter phagocyte function,
decrease activation of the inflammatory cytokine nuclear factor-κB, and reduce platelet and T-cell
adhesion. Their experiments also showed that phages can
inhibit the formation of reactive oxygen species by neutrophils,
which reduces tissue damage that accompanies the chronic inflammatory process.
During their study, the researchers also observed a significant decrease in the
level of C-reactive protein (an inflammatory marker). All of these
anti-inflammatory properties contribute to reduction of symptoms.
Carmody and colleagues also describe anti-inflammatory effects of
phages in their research on Burkholderia cenocepacia infection. In order to determine whether
phage treatment could attenuate infection-associated lung inflammation, levels
of proinflammatory cytokines TNF-α and MIP-2 were measured in the
lungs forty-eight hours after treatment. In the AU0728 infected mice, it was
observed that treatment with phage resulted in significantly reduced TNF-α level, whether administered via
inhalation or intraperitoneal injection. It was also found that mice treated via
intraperitoneal injection had significantly reduced
levels of MIP-2 in the lungs. To access the proinflammatory
potential of phage alone, cytokine levels in mock-infected mice were measured after
phage treatment twenty-four hours later. These mice showed no appreciable
levels of either TNF-α or MIP-2 in the lungs forty-eight hours after
treatment.
Alternative Phage Treatments
In
addition to the study of the efficacy of phages in the treatment of bacterial
infections, notable research has been conducted in the study of the
effectiveness of combinations of phage and antimicrobial therapy, as well as
the use of lysins produced by phages to directly
combat pathogenic bacteria.
Zimecki and colleagues describe the benefits of combined action of lactoferrin and phages in the treatment of infection of Escherichia coli and S. aureus in mice (12). Lactoferrin (LF) is a protein found in the secretory fluids of mammals and the secondary granules of neutrophils, and exhibits both antibacterial and anti-inflammatory actions. It has also been shown to enhance neutrophil production. In the experiment, mice were injected with E. coli and S. aureus, while the E. coli and S. aureus specific bacteriophages were administered intraperitoneally or orally one hour after bacteria injection. Lactoferrin was injected intravenously 24 hours before infection. The results showed that both oral and intraperritoneal phage administration proved effective in reducing numbers of bacteria in the liver, but the combined action of bacteriophages and LF produced a significant additive effect.
Fischetti and colleagues studied the antibacterial effect of bacteriophage lysins in various
types of infections (5). Lysins, the phage protein
that cause bacteria to rupture, or “lyse”, by
breaking down their pepdidoglycan cell wall, can kill
gram-positive bacteria seconds after contact. These researchers studied the
efficacy of lysins in the treatment of different
diseases as well as their potential to be used synergistically with
antibiotics. In contrast to studies done with phages, this study found that one
dose of lysin administered one hour after infection
with S. pneumonia was not enough to
fully eliminate the bacteria after 48 hours. However, when treatment was
initiated at 24 hours and every 12 hours thereafter, 100% of mice recovered
from otherwise fatal pneumonia. Since lysins cannot
reproduce like phages, multiple enzyme doses or a constant infusion of enzyme
is required to completely eliminate infection.
Safety of Phage Therapy
Although bacteriophages are generally regarded as safe, there are a few concerns that are addressed by the literature. Some concerns address some of the same problems associated with antibiotics, such as acquired resistance, while other concerns are directed exclusively at the safety of phages for use as a therapeutic agent.
Concerns with Lytic
Activity
In their
introduction to the potential of phage therapy in the treatment of infections, Kropinski and colleagues introduced the concern over some
phages, called temperate phages, being able to potentially transfer virulent
genes between strains of bacteria, a process called “lysogenic
conversion” (6). Temperate phages undergo the lysogenic
cycle to integrate their DNA into the bacterial DNA, which is then replicated
in each bacterial daughter cell.
Virus DNA can sometimes carry genes that increase bacterial pathogenicity. Also, infection by temperate phages does not
always lead to propagation and cell lysis so this
type of phage does not kill 100% of bacteria. Therefore, only lytic phages, where infection leads exclusively to cell
death and lysis by the lytic
cycle, have the potential to be used as a therapeutic agent.
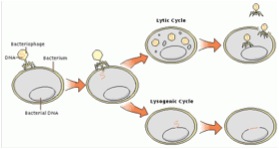
Figure 2. Bacteriophage Lysogenic
and Lytic Cycle. Retrieved from <http://www.mining.ubc.ca/cerm3/>.
The direct
killing and lysis of bacteria by lytic
phages, however, brings up new concerns in the safety of this therapeutic
agent. Matsuda and colleagues addressed the role that phage therapy can have in
the increased expression of inflammatory mediators which
can lead to dangerous complications in patients (9). Lysis
of some species of bacteria can lead to endotoxin
release, which causes a tremendous increase in pro-inflammatory cytokine
production, possibly resulting in toxic shock. Endotoxin
release is also a potential problem with antibiotics that cause lysis. These researchers studied the efficacy of mutant A3
T4 (LyD) phages, which do not express holin, a protein needed to lyse
bacterial cell walls. Since these are lytic phages,
infection will always lead to cell death, but since they lack holin, should not lead to cell lysis.
A study was conducted on mice with peritonitis caused by E. coli. Mice injected with LyD phages
showed a significant survival advantage, with 81% survival in 48 hours. These
findings were compared to control mice (0%) and wild type phages (52%). LyD phage therapy decreased endotoxin
levels as well as cytokine release.
Immune Response
In
contrast to the positive effects on the immune system that phages showed in
some studies, there has been a concern over a possible negative immune response
to phages, especially after repeated treatment. An acquired immune response to
phages or their derivatives through antibodies can severely limit the treatment
potential of these viruses.
In the
study conducted by Fischetti on lysins,
the effects that antibodies have on the therapeutic effects of phage particles
was examined. Since lysins are proteins that can
stimulate an immune response, activation by the immune system could show a
significant interference in the therapeutic effect, something that is not a
problem in traditional antibiotics. To investigate this problem, rabbit hyperimmune serum raised against the pneumonia-specific
phage enzyme Cpl-1 was tested for its effect on lytic
activity. The results of the study found that highly immune serum slows, but
does not block the lytic activity of Cpl-1. A study
was then conducted on mice that tested positive for antibodies against Cpl-1
from a previous inoculation with the phage. After intravenous inoculation of pneumococci, mice with the antibody against Cpl-1 showed
the same degree of reduction of bacteria as naïve mice, indicating that lysin antibodies have little to no neutralizing effect.
In their
research article on Burkholderia cenocepacia
pulmonary infection, Carmody and colleagues suggest
that the effect of phage therapy is rapid and likely faster than specific
immunity can develop. However, the effect of long-term exposure to circulating
phage is unknown. Repeated dosing using phages with different antigens to avoid
immunologic reactivity is a possible means to circumvent this problem. Most
scenarios in clinical settings would involve cocktails of multiple phages with
different receptor specificities.
Conclusion
The
literature reviewed in this article has contributed to the understanding of the
potential, as well as the present obstacles in bringing phage therapy into a
mainstream reality. These articles present impressive research on the efficacy
of phage therapy, as well as issues that need to be addressed for future
studies. The results of the study on the effectiveness of phage therapy look
encouraging in both direct lytic activity and immune
system enhancement. The safety of this method raises some issues, and more
research must be done before this therapy can gain wide acceptance for use in
humans.
Most
research that has been conducted formally has been on mice, and human studies
and treatment remain exclusive to Eastern Europe. It will be a major challenge
for phage therapy to take hold in western medicine because it challenges the
conventional view of a “drug.” Unless pharmaceutical companies can make a sure
profit and regulatory approval, many will be unwilling to invest in this
technology.
By taking
small steps in that direction, however, this technology can someday take hold
in western medicine. Regulatory approval might be difficult for “live” viruses,
but the use of bacteriophage lysins
in the control of bacterial infections could gain easier acceptance. Continued
research on new alternative therapies such as this will help expand our
treatment options beyond antibiotics.
References
1. Abhilash, M., Vidya, A.G., Jagadevi, T. (2009). Bacteriophage
Therapy: A War Against Antibiotic Resistant Bacteria. The Internet Journal of Alternative
Medicine. 7 (1).
2. Borysowski, J.. (2008, Sep). Is phage therapy acceptable in the immunocompromised host?. International Journal of Infectious Diseases. 12(5), 466-471. from Science Direct.
3. Carmody, L. A. (2010, Jan). Efficacy
of bacteriophage therapy in a model of Burkholderia cenocepacia
pulmonary infection. The Journal of
Infectious Diseases. 201(2), 264-271. from Medline.
4. Chhibber, S. (2008). Therapeutic
potential of bacteriophage in treating Klebsiella pneumoniae
B5055-mediated lobar pneumonia. Journal of Medical Microbiology, 57 pp.
1508-1513.
5. Fischetti, V. A. (2008, Oct). Bacteriophage lysins
as effective antibacterials. Current Opinions in Microbiology. 11(5),
393-400. from PubMed.
6. Kropinski, A. M. (2006, Sep). Phage
Therapy- Everything Old is New Again. Medical Microbiology. 17(5),
297-306. from PubMed.
7. Letkiewicz,
S.. (2010, Nov). The perspectives of the application of phage therapy in chronic
bacteria prostatitis. Fems
Immunology and Medical Microbiology. 60(2), 99-112.
from ISA Web of Knowledge.
8. Levin,
B., Bull, J. (2004). Population and Evolutionary Dynamics of
Phage Therapy. Nature Review:
Microbiology. (2), 166-173.
9. Matsuda,
T.. (2005, Jun). Lysis-deficient bacteriophage
therapy decreases endotoxin and inflammatory mediator
release and improves survival in a murine peritonitis
model. Surgury. 137(6),
639-546. from ScienceDirect.
10. Sulakvelidze, A.. (2005, Jun). Phage therapy: an
attractive option for dealing with antibiotic-resistant bacterial infections . Drug Discovery Today.
10(12), 807-809. from ScienceDirect.
11. Zimecki,
M.. (2009, Aug). Effects of
prophylactic administration of bacteriophages to immunosuppressed mice infected with Staphylococcus aureous. BMC Microbiology. 17(9),
169-. from PubMed.
12. Zimecki, M.. (2008,
Feb). The concerted action of lactoferrin and bacteriophages in the clearance of bacteria in sublethally infected mice. Postepy Hig Med Dosw. 7(62), 42-46. from PubMed.
If you would like to see previous comments or leave comments about this website, click on the comment button below.
